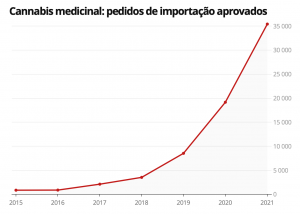
Import requests for cannabis derivatives in Brazil increased by more than 2400%, from 850 in 2015 to more than 35 in 2021, according to data from Anvisa – National Health Surveillance Agency. In 2022, until the beginning of October, 58.245 requests for imports of cannabis-derived products for therapeutic purposes were processed in Brazil, which confirms that medical cannabis in Brazil is booming.
Requests for imports of cannabis derivatives for therapeutic purposes continue to increase in Brazil, revealing an average annual growth of around 400%, figures that could expand even further until the end of this year. If in 2015 there were 850 import requests, that number skyrocketed to 35.416 in just 6 years. The recent election of Lula da Silva as the new president of Brazil now brings new hope to the sector, because it could represent advances in the regulation of cannabis in Brazil, something totally unthinkable for the previous government of Jair Bolsonaro.
Lula did not express himself clearly in favor of legalization, but he also did not say he was against it, referring this decision to other instances than the government: “This is not an issue that the government has to deal with. This is an issue that either the National Congress handles, or the Supreme Court takes care of it,” he told reporters. flow podcast.
Source: Anvisa
Brazilian civil society has, for its part, been extremely active, having even overturned a CFM resolution – Federal Council of Medicine, which intended to limit the use and prescription of medical cannabis in the country only to cannabidiol.
In contradiction with Anvisa itself, which had published in October 2021 a product list cannabis derivatives approved for import, CFM received fierce criticism de various quadrants Brazilians and ended up revoking the Resolution days after its publication.
A Anvisa approved in 2021 the import of 249 cannabis derivatives into Brazil, of which five with THC and 244 based on CBD (cannabidiol). The authorized products followed a new resolution, with simpler rules for individual importation by Brazilian patients, but Anvisa clarified at the time that “it did not evaluate the effectiveness, quality or safety of the products”, referring to the legislation of the countries of origin.































